Adaptation and failure of pancreatic beta cells in health and in diabetes
The Wortham Lab employs metabolomics, single cell genomics, and islet functional studies in human pancreatic tissue and in genetically modified mice to understand how signals from the systemic and local environment impact the pancreatic beta cell during homeostasis and in diabetes.
How do beta cells anticipate the amount of insulin required for the next meal?
Current projects
Our prior work has demonstrated that beta cells exhibit a transient “memory” of prior nutrient state—encoded in part by histone modifications—that influences the strength of future insulin secretory responses. For example, we found that when beta cells cannot “reset” the histone modifications associated with meal feeding they do not appropriately recognize the fasted state between meals, and as a result they oversecrete insulin. We are now studying how this mechanism regulates insulin secretion during insulin resistance and in type 2 diabetes. Ongoing work in the lab will also define the cell signaling cascades that couple environmental nutrient signals to histone modifications.
To learn more, read our paper on epigenomic regulation of adaptive insulin secretion in Journal of Clinical Investigation and associated commentary article.
How does excessive workload cause beta cell failure in type 2 diabetes?
The amount of insulin required per beta cell is termed “beta cell workload”. Workload is a constant pressure that the beta cell must cope with, and conditions such as insulin resistance can chronically increase this pressure. While beta cells are equipped with adaptive mechanisms that allow them to compensate for increased workload, their capacity to adapt is not unlimited. Understanding the biological basis for these limitations and the mechanisms underlying beta cell failure when they do reach a “breaking point” could reveal therapeutic strategies for expanding or sustaining the beta cell’s capacity to meet insulin requirements.
To learn more, watch Matt’s SugarScience talk on beta cell failure in T2D.
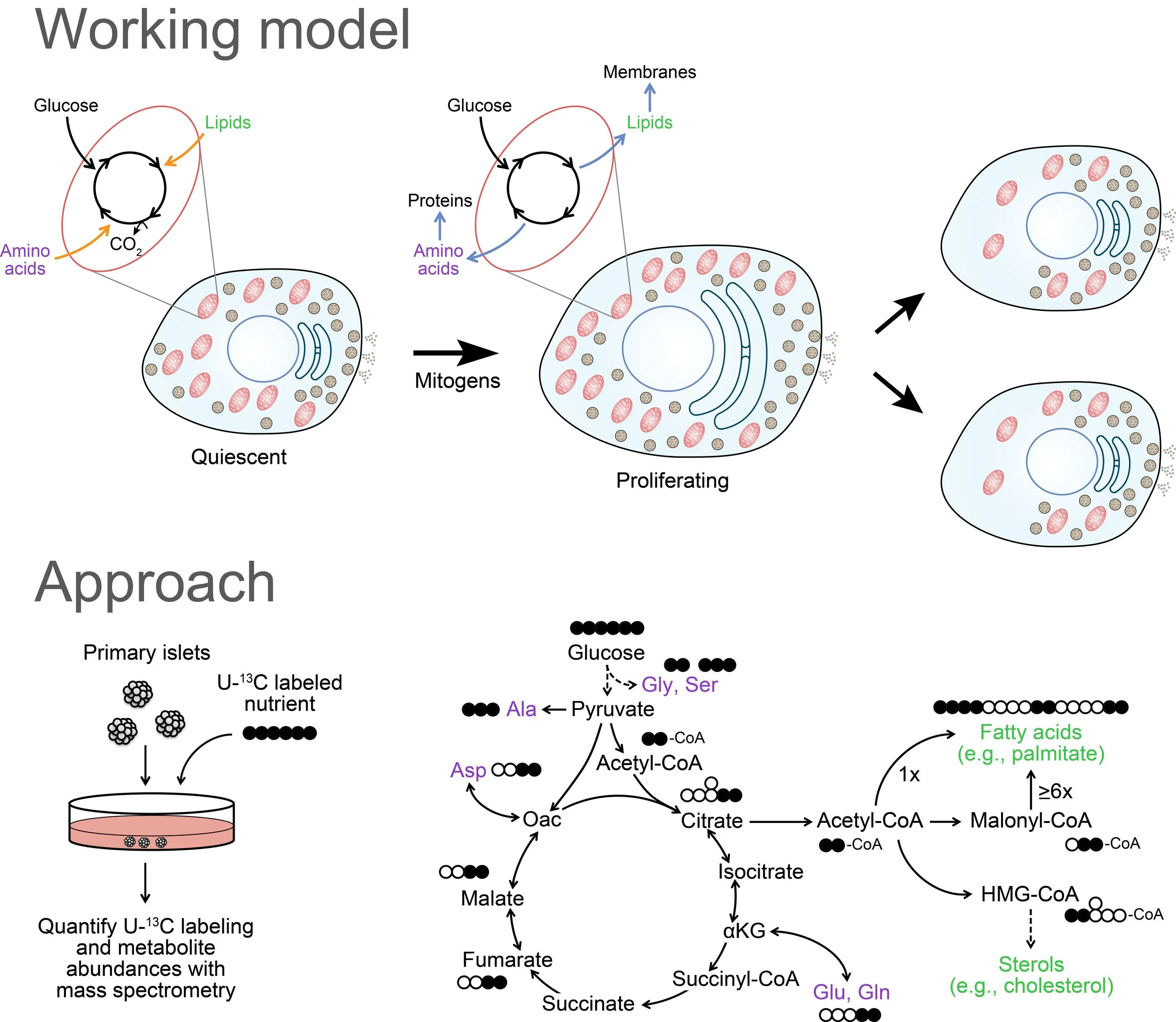
Does the unique metabolic program of the beta cell restrain its ability to regenerate?
Beta cells couple insulin secretion to systemic glucose by metabolizing this nutrient at a rate proportional to its concentration in the blood. Therefore, beta cells are dedicated glucose sensors that are limited in their ability to shut off or reroute glucose metabolism. Studies of proliferating cells in other contexts indicate that remodeling of metabolism is necessary for cells to meet the biosynthetic demands of cell growth and division. While the diabetes field has made a heroic effort to correct a shortfall of beta cells by stimulating their proliferation, even the most successful agents coax fewer than 10% of beta cells out of quiescence. Ongoing work in the lab will test the hypothesis that the nutrient-sensing metabolic program is a major bottleneck to therapeutic beta cell expansion.
To learn more, read about our Human Islet Research Network New Investigator Award as well as our paper on metabolic regulation of adaptive proliferation in Journal of Clinical Investigation and the associated commentary article.